Facilities
- Home
- Facilities
- Misato Factory
Features
- The Misato Factory has acquired GMP certification from overseas health authorities, including the US FDA, ANVISA, and Russian Ministry of Health.
- Pharmaceutical products manufactured at this factory are shipped to more than 50 countries through our customers.
- The Factory is competent to provide contract services for manufacturing of solid as well as injectable dosage forms on a large scale with various models of automated equipment.
- The Misato Cold Chain Center is competent to provide contract services of inspection, packaging, and storage of biological and regenerative medicine products at extremely low temperatures.
History
Our plant was constructed in 1981 by Eisai Co., Ltd.
Scale and equipment
The site area is approximately 173,000 square meters, which includes the space for the Plant area where pharma are manufactured, as well as separate space for welfare facilities (gymnasium, playing field, etc.).
The total floor space of the plant is approximately 57,000 square meters, consisting of a solid dosage production building, an injectables building, a warehouse building and a QC area. Our Misato plant features a wide range of large scaled manufacturing equipment, allowing our solid dosage manufacturing capacity to be approximately twice that of the Kawagoe facility.
Layout
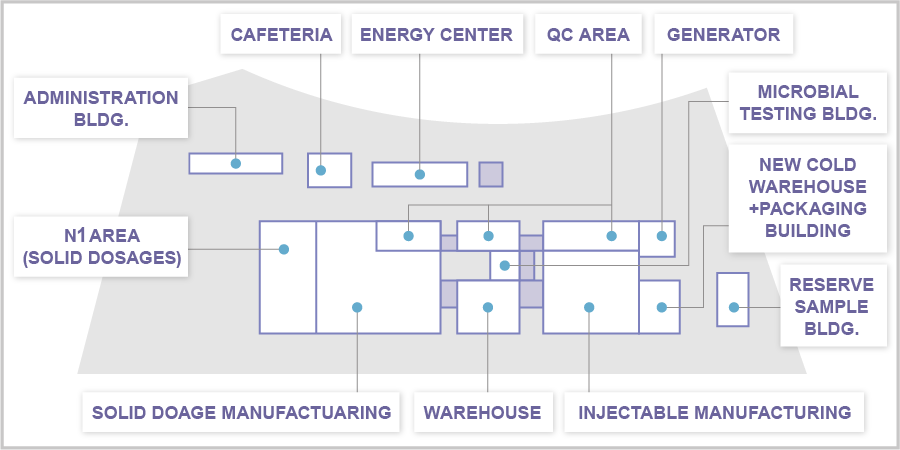
Solid Dosage Formulation Area
(N1 Formulation Building, N1 Work Room )
Manufacturing
Solid dosage forms (uncoated tablets, film-coated tablets, sugar-coated tablets, granules, and dry syrups)
Injectables (vials, syringes, lyophilized formulation)
PTP, bottle, and stick packaging
Inspection of injectables and secondary packaging
Management of Formulation Buildings
Dedicated air-conditioning equipment: All formulation buildings are equipped with dedicated air-conditioning and ventilation equipment.
Prevention of contamination: Contamination is prevented through control of pressure difference between work rooms and clean corridors, and the working environment is controlled and maintained in optimal conditions.
Manufacturing areas: The area environment is controlled and maintained at the cleanliness class of 100,000, room temperature between 17 and 28°C, and humidity between 30% and 65% RH.
Tableting and coating machines: The machines are installed for efficient manufacturing of products mainly at night.
Packaging area
Filling area for primally packaging
Secondly packaging area
Management of Packaging Facilities
The pressure difference in the working environment of the packaging facilities is adequately controlled to maintain the environment as clean and free of contamination.
Injectable dosage formulation area
(N2 Formulation Building)
In-area capacity
Manufacture to packaging
- · The manufacture of ampule
- · The manufacture of vial
- · The manufacture of pre-filled syringe
- · The manufacture of freeze-dried pharmaceuticals
Manufacturing room
Liquid pharmaceuticals, filling and sterilization (except for aseptic packaging)
Humidity, temperature and pressure differences, air speed and hygiene grade are intensively controlled
Monitored using a round-the-clock monitoring system
Light blocking functionality is in place
Work rooms are available for the manufacture of vial and lyophilized products with aseptic filling technologies.
Storage (J center)
J Center (Warehouse)
A manufacturing support facility where raw materials, packaging materials, and products are brought into and dispatched from
Large-scale racks
The capacity: To store 3,860 pallets
Ordinarily air-conditioned warehouse
Specially air-conditioned warehouse
Products, raw materials and excipients requiring air-conditioning are stored in the specially air-conditioned racks and maintained
System
Operating management system
Automatic transport system
Transport within the warehouse: Completely automated unmanned system
Refrigerated warehouse
A storage space aside for hazardous substances
The Misato Cold Chain Center
History and scale
This 3,900 square meter cold chain center was established in September 2020 at the Misato Plant.
It is used as a storage warehouse for sterile drug products and biologics that require refrigerated storage management. The center is an important hub for Bushu Pharma's "GATEWAY to ASIA®" strategy, enabling the company to receive bulk products from customers, conduct quality inspection, labeling, packaging, and supply to Japan, other Asian countries, and the rest of the world.
features
As of October 2023, the Factory has a housing capacity of 300 pallets.
Work items
Packaging under refrigerated conditions
General secondary packaging
Packaging of clinical trial supplies
Precise temperature control appropriate for biological products, regenerative medicine products, vaccines, etc. from receipt to delivery
CONTACT
As a company specializing in contract manufacturing of pharmaceutical drugs, we offer a wide range of contract services tailored to clients’ needs based on our extensive experience and know-how accumulated over many years.



