Facilities
- Home
- Facilities
- Kawagoe Factory
Features
- The Kawagoe Factory has acquired GMP certification from major overseas health authorities, including the US FDA, ANVISA, Health Canada, and EMA (including the VMD ).
- The Factory is competent to provide a broad spectrum of contract services, from the manufacture of clinical trial supplies and commercial products in both small and large scales, from the stage of research and development to the inspection and packaging of biological and regenerative medicine products that must be strictly controlled at extremely low temperatures.
- Our Medical Device Center has the capacity to inspect and package medical devices and drug-device combination products*.
* Drug-device combination products: Products comprising both medical devices and pharmaceuticals (including biological medicines)
History
Our plant was constructed in 1981 by Sandoz Yakuhin, the forerunner of the present Novartis Pharma, designed and supervised based on a European concept.
Scale and equipment
The plant site covers around 65,000 m², with a total floor area of around 41,000 m², and offers plenty of scope for future expansion.
Branching off from a roughly 180 m long central corridor are interconnected buildings for manufacturing, packaging, warehousing, engineering/maintenance, and QC testing. We also have a separate Medical Device Center.
Layout
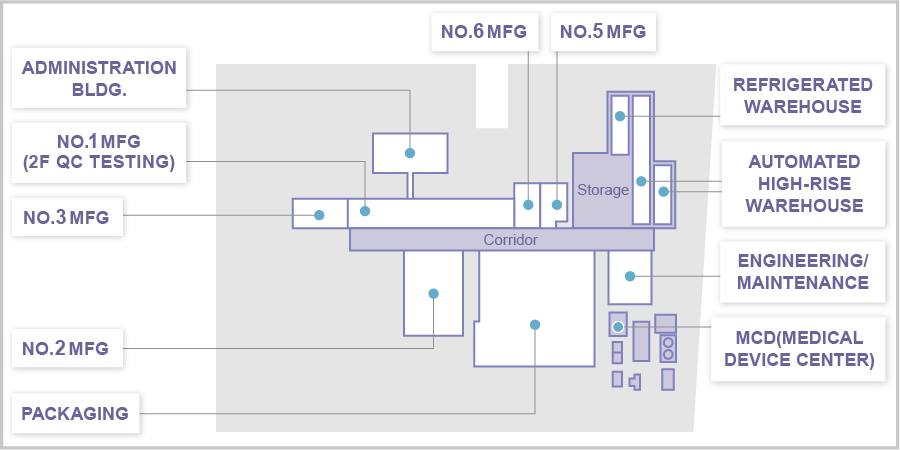
Manufacturing Buildings
No.1 Mfg: General Manufacturing
No.2 Mfg: General Manufacturing
No.3 Mfg: Hormone Manufacturing, Packaging
No.4 Mfg: General Manufacturing
No.5 Mfg: High Potency Drug Manufacturing
Management of Formulation Buildings
Dedicated air-conditioning equipment: All formulation buildings are equipped with dedicated air-conditioning and ventilation equipment.
Prevention of cross-contamination: Cross-contamination is prevented through control of pressure difference by shifting the pressure in clean corridors toward positive and that in the production room toward negative.
Manufacturing areas: The environment of the areas is controlled and maintained at the cleanliness class of 100,000, room temperature between 18 and 28°C, and humidity between 40% and 60% RH (with certain exceptional conditions).
The N2 Formulation Building: Manufacturing operations have been improved to be more productive by introducing vertical flow systems (vertical transport lines) for unmanned, automated operations and automatic transport systems at night.
Kawagoe Factory Packaging
Solid Pharmaceuticals
Primary Packaging Area
Secondary Packaging Area
Injectables
Secondary Packaging Area
Management of Packaging Facilities
Primary packaging area: The area is controlled and maintained at the cleanliness class of 100,000, room temperature between 18 and 28°C, and humidity between 40% and 60% RH (with certain exceptional conditions).
Secondary packaging area: The area is controlled and maintained at room temperature between 18 and 28°C (with certain exceptional conditions).
Packaging facilities are divided by partitions to prevent cross-contamination and to organize the working environment for a leaner and cleaner way of working.
Storage
Automated High-Rise Warehouse 1
Automated High-Rise Warehouse 2
Refrigerated Warehouse
Management of Warehouse Buildings
The warehouse buildings are managed with computer-controlled automatic high-rise warehouses, automated guided-vehicle (AGV) systems, and logistics systems designed to automatically distribute products and materials to warehouses, corridors, and manufacturing buildings.
Cold storage warehouses are equipped with emergency backup power supplies for use in case of unexpected interruption of power or outages.
The buildings are also equipped with storage depots for hazardous materials.
Specifications of each storage
| Temperature Management | Pallet Capacity | Stored Items | |
|---|---|---|---|
| Automated High-Rise Warehouse 1 | 8℃ to 25℃ | 3,400 | Raw materials, semi-finished products, final products, materials |
| Automated High-Rise Warehouse 2 | 1℃ to 30℃ | 1,900 | |
| Refrigerated Warehouse | 2℃ to 8℃ | 498 | All products requiring refrigeration |
-
Automated High-Rise Warehouse 1
- Temperature Management
- 8℃ to 25℃
- Pallet
Capacity - 3,400
- Stored Items
- Raw materials, semi-finished products, final products, materials
-
Automated High-Rise Warehouse 2
- Temperature Management
- 1℃ to 30℃
- Pallet
Capacity - 1,900
- Stored Items
- Raw materials, semi-finished products, final products, materials
-
Refrigerated Warehouse
- Temperature Management
- 2℃ to 8℃
- Pallet
Capacity - ~500
- Stored Items
- All products requiring refrigeration
Medical Device
Center
Performed acceptance tests and packaging work for medical equipment and combination products*
*combination products of pharmaceuticals and medical devices
CONTACT
As a company specializing in contract manufacturing of pharmaceutical drugs, we offer a wide range of contract services tailored to clients’ needs based on our extensive experience and know-how accumulated over many years.



